UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
November 20, 2019
Date of Report (Date of earliest event reported)
Bicycle Therapeutics plc
(Exact name of registrant as specified in its charter)
|
England and Wales |
|
001-38916 |
|
Not applicable |
|
(State or other jurisdiction |
|
(Commission |
|
(IRS Employer |
|
B900, Babraham Research Campus |
|
Not Applicable |
|
(Address of principal executive offices) |
|
(Zip Code) |
Registrant’s telephone number, including area code: +44 1223 261503
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
o Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Securities registered pursuant to Section 12(b) of the Act:
|
Title of each class |
|
Trading Symbol(s) |
|
Name of each exchange on which registered |
|
Ordinary shares, nominal value £0.01 per share |
|
n/a |
|
The Nasdaq Stock Market LLC* |
|
American Depositary Shares, each representing one ordinary share, nominal value £0.01 per share |
|
BCYC |
|
The Nasdaq Stock Market LLC |
* Not for trading, but only in connection with the listing of the American Depositary Shares on The Nasdaq Stock Market LLC.
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§ 230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§ 240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. x
Item 7.01 Regulation FD Disclosure
On November 20, 2019, Bicycle Therapeutics plc (the “Company”) posted an updated corporate presentation on the Company’s website. In such presentation, the Company announced key events it expects to occur in 2020. The Company expects to initiate a Phase IIa clinical trial of its BT1718 product candidate, provide interim data regarding its Phase I clinical trial of BT5528, initial a Phase I clinical trial of BT8009, and advance Investigational New Drug application enabling activities regarding BT7480 in 2020. A copy of the presentation is furnished as Exhibit 99.1 to this Current Report on Form 8-K and is incorporated herein by reference.
The information furnished under this Item 7.01, including Exhibit 99.1, shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended, or subject to the liabilities of that section. The information shall not be deemed incorporated by reference into any other filing with the Securities and Exchange Commission made by the Company, regardless of any general incorporation language in such filing.
Item 9.01 Financial Statements and Exhibits
|
Exhibit No. |
|
Description |
|
99.1 |
|
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
|
Date: November 20, 2019 |
BICYCLE THERAPEUTICS PLC | |
|
|
|
|
|
|
By: |
/s/ Lee Kalowski |
|
|
Name: Lee Kalowski | |
|
|
Title: Chief Financial Officer | |
Forward-looking statements Nov-2019 2 This presentation may contain forward-looking statements made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. These statements may be identified by words such as “aims,” “anticipates,” “believes,” “could,” “estimates,” “expects,” “forecasts”, “goal,” “intends,” “may” “plans,” “possible,” “potential,” “seeks,” “will,” and variations of these words or similar expressions that are intended to identify forward-looking statements. All statements other than statements of historical facts contained in this presentation are forward-looking statements, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters; our current and prospective product candidates, planned clinical trials and preclinical activities, current and prospective collaborations and the timing and success of our development of our anticipated product candidates. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our preclinical and clinical results and other future conditions, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to, the risk that any one or more of our product candidates will not be successfully developed or commercialized, the risk of cessation or delay of any ongoing or planned clinical trials, the risk that we may not realize the intended benefits our technology, including that we may not identify and develop additional product candidates for our pipeline, the risk that we may not maintain our current collaborations or enter into new collaborations in the future, or that we may not realize the intended benefits of these collaborations, the risk that our product candidates or procedures in connection with the administration thereof will not have the safety or efficacy profile that we anticipate, the risk that prior results will not be replicated or will not continue in ongoing or future studies or trials, the risk that we will be unable to obtain and maintain regulatory approval for our product candidates, the risk that the size and potential of the market for our product candidates will not materialize as expected, risks associated with our dependence on third-parties, risks regarding the accuracy of our estimates of expenses, risks relating to our capital requirements and needs for additional financing, and risks relating to our ability to obtain and maintain intellectual property protection for our product candidates. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause our actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in our Quarterly Report on Form 10-Q, filed with the Securities and Exchange Commission (SEC) on November 7, 2019, as well as in other filings Bicycle may make with the SEC in the futurer , as well as discussions of potential risks, uncertainties and other important factors in our subsequent filings with the Securities and Exchange Commission. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. Except as required by applicable law, we do not plan to publicly update or revise any forward-looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise.

We aim to redefine what’s possible for people with cancer and other serious diseases by pioneering a new and differentiated class of innovative treatments Developing world’s first fully synthetic immuno-oncology (IO) platform Led by world class management team, supported by strong financial foundation Nov-19 3 Our goal is to create transformational medicines Advancing wholly-owned oncology pipeline of multiple clinical assets Engineering Bicycles® to solve unique therapeutic challenges Exploring broad potential beyond oncology through partnerships

Bicycles® are a new therapeutic modality for addressing intractable challenges 4 X X X Short linear peptide Built-in tolerance to conjugation Generalizable approach Versatility to adopt multiple formats Phage-based screening platform Nobel Prize-winning technology Rapid selection from >1017 potential candidates Robust patent protection >90 patent families Expiration into 2030s Nov-19 Bicycle Chemical modification with scaffold Scaffold Chemical synthesis Rapid tissue distribution Complex protein targets druggable Route of elimination Small molecules +++ +++ --- Liver Antibodies --- + +++ Liver Bicycles +++ +++ +++ Renal

Leverage platform’s versatility More efficiently bring novel medicines to patients ONCOLOGY BEYOND ONCOLOGY Internal focus on oncology, partnerships to explore additional applications across therapeutic areas Nov-19 5 Bicycle® Toxin Conjugates Bicycle Tumor-targeted Immune Cell Agonists Toxin delivery system with unique mechanism of action Potentially improved safety & efficacy over other modalities Differentiated approach to agonizing immune cells May overcome limitations of antibody & biologic therapies
Product/Target Therapeutic Interest Collaborator Collaborator Stage Stage Stage Discovery/ Preclinical Discovery/ Preclinical Clinical Bicycle® Toxin Conjugates Discovery/Preclinical Phase 1 BT1718 (MT1-MMP) Oncology BT5528 (EphA2) Oncology BT8009 (Nectin-4) Oncology Bicycle Tumor-targeted Immune Cell Agonist (TICAs™) BT7480 (Nectin-4/CD137 TICA) Oncology EphA2/CD137 TICA Oncology Beyond Oncology THR-149 (Kallikrein inhibitor Bicycle) Ophthalmology Inhaled Bicycles Respiratory Novel anti-bacterials Anti-infectives Novel CNS targets CNS diseases Robust proprietary and partnered pipeline 6 Nov-19

Bicycle® Toxin Conjugates

8 Specific tumor targeting via antigen Release of toxin directly into tumor via cleavable linker Large amount of cytotoxic payload can be delivered Bicycle® Toxin Conjugates – designed to be selective tumor targeting therapeutics MWt of 1.5-2kDa, 50-100x smaller than antibodies Rapid tumor penetration Renal elimination enhances tolerability Short terminal half-life Flexible dosing (mono or combo therapy) Nov-19 TOXIN LINKER BTCs offer advantages over antibody drug conjugate (ADC) and small molecule approaches Antibody Bicycle 0.001 0.010 0.100 1.000 0 12 24 36 48 60 72 [Tissue] ( uM ) Time (h) BTC plasma Toxin tumor Toxin plasma

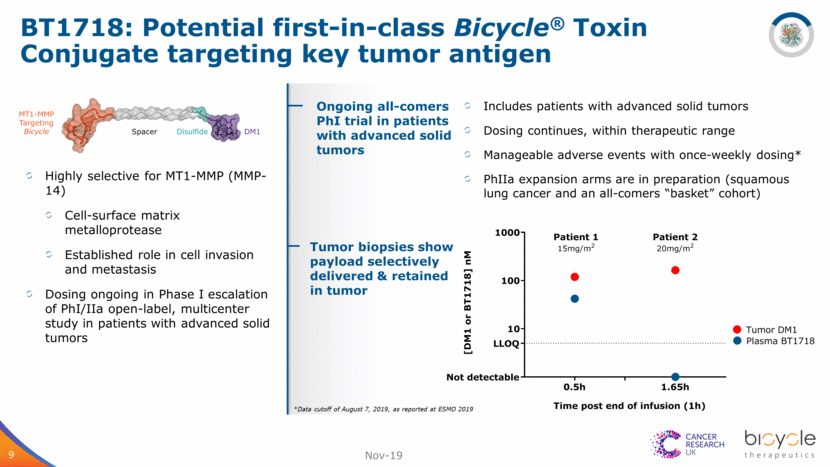
BT1718: Potential first-in-class Bicycle® Toxin Conjugate targeting key tumor antigen 9 Highly selective for MT1-MMP (MMP-14) Cell-surface matrix metalloprotease Established role in cell invasion and metastasis Dosing ongoing in Phase I escalation of PhI/IIa open-label, multicenter study in patients with advanced solid tumors Nov-19 Ongoing all-comers PhI trial in patients with advanced solid tumors *Data cutoff of August 7, 2019, as reported at ESMO 2019 Includes patients with advanced solid tumors Dosing continues, within therapeutic range Manageable adverse events with once-weekly dosing* PhIIa expansion arms are in preparation (squamous lung cancer and an all-comers “basket” cohort) Tumor biopsies show payload selectively delivered & retained in tumor 0.5h 1.65h 10 100 1000 [ D M 1 o r B T 1 7 1 8 ] n M LLOQ Not detectable Tumor DM1 Plasma BT1718 Patient 1 15mg/m 2 Patient 2 20mg/m 2 Time post end of infusion (1h)
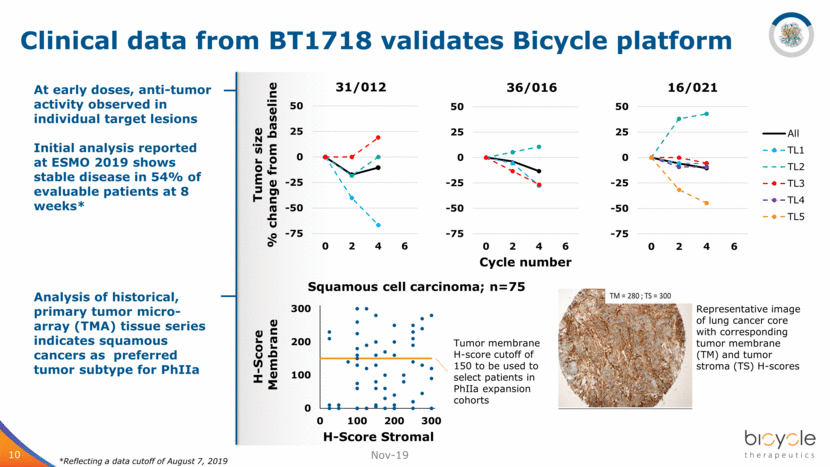
Clinical data from BT1718 validates Bicycle platform Nov-19 10 At early doses, anti-tumor activity observed in individual target lesions Initial analysis reported at ESMO 2019 shows stable disease in 54% of evaluable patients at 8 weeks* Analysis of historical, primary tumor micro- array (TMA) tissue series indicates squamous cancers as preferred tumor subtype for PhIIa Tumor size % change from baseline Cycle number Representative image of lung cancer core with corresponding tumor membrane (TM) and tumor stroma (TS) H-scores *Reflecting a data cutoff of August 7, 2019 Tumor membrane H-score cutoff of 150 to be used to select patients in PhIIa expansion cohorts 0 100 200 300 0 100 200 300 H - Score Membrane H - Score Stromal Squamous cell carcinoma; n=75
BT5528: EphA2 BTC delivers improved preclinical safety and efficacy over comparator ADCs 11 EphA2 is overexpressed in many difficult to treat tumors Previous antibody drug conjugate projects, notably MEDI-547, have failed due to bleeding events Preclinical models are predictive Findings from BT5528 GLP tox study show no coagulation problems at toxin equivalent doses >150x clinical dose of MEDI-547 used in patients Dosing is underway in Phase I/II open-label, multicenter study in patients with advanced solid tumors evaluating BT5528 as a monotherapy and in combination with nivolumab Nov-19 Bicycle® distribution at 60 min ADC distribution at 60 min Displayed superior activity to EphA2 ADC in large tumor xenografts Docetaxel resistant NSCLC model No signs of coagulopathy or bleeding 0 7 14 21 28 35 42 49 56 63 70 77 84 91 98 0 1000 2000 3000 4000 ^ ^ ^ Days after start of dosing T u m o r v o l u m e ( m m 3 ) Vehicle QW BT5528 3mg/kg QW ADC 3mg/kg QW ^ time of administration ^ ^ ^ ^

BT8009: Nectin-4 BTC fast follower with differentiated profile to clinical ADC 12 Nectin-4 is involved in establishing cell-cell contact and tumor cell survival, overexpressed in common types of cancer (e.g. bladder, breast, gastric, lung and ovarian) Target validated in the clinic by enfortumab vedotin (Astellas/SeaGen) BT8009 designed to avoid hepatic exposure, may overcome stromal barrier in pancreatic cancer, easily manufactured IND-enabling studies ongoing Nov-19 Improved activity over Nectin-4 ADC Impressive selective delivery of toxin to tumor 0 7 14 21 0 200 400 600 800 1000 Days after start of dosing T u m o u r v o l u m e ( m m 3 ) ^ ^ ^ ADC 4mg/kg QW Vehicle QW BT8009 5mg/kg QW BT8009 3mg/kg QW ^ time of administration 0 24 48 72 1 10 100 1000 10000 Time (h) [ M M A E ] p m o l / m l o r p m o l / g MMAE tumor BT8009 plasma MMAE plasma

Bicycle® Tumor-targeted Immune Cell Agonists (TICAsTM)

Plug and play for flexible therapeutic design Properties of Bicycles® are uniquely suited to IO modulation 14 Nov-19 Designed for optimal immune cell engagement Rapid tumor penetration Renal elimination avoids liver toxicity Small molecule COGS Short half-life Flexible dosing (mono or combo therapy) Tuneable PK to vary degree of activation Fully synthetic Generalizable format Specific tumor targeting via antigen binding Bicycle Bicycle targeting immune cell agonist receptor Bicycle TICAsTM enable optimum spacing compared to bulkier biologics

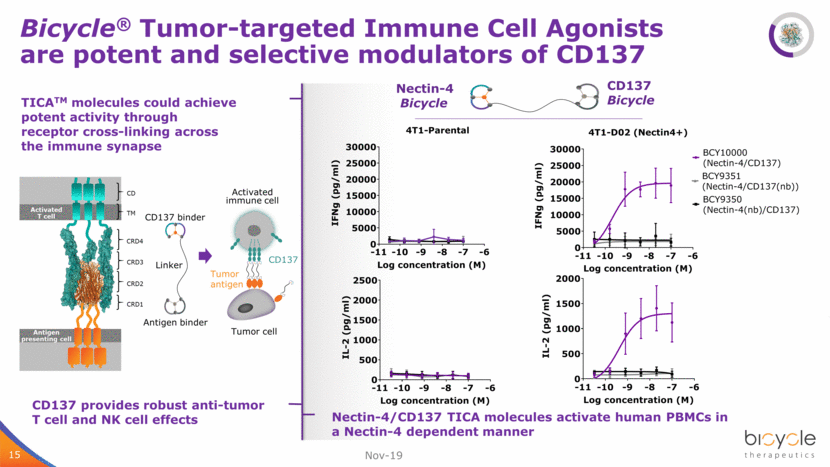
15 Bicycle® Tumor-targeted Immune Cell Agonists are potent and selective modulators of CD137 15 TICATM molecules could achieve potent activity through receptor cross-linking across the immune synapse CRD1 CRD2 CRD3 TM CD Antigen presenting cell Activated T cell CRD4 Tumor antigen CD137 Tumor cell Activated immune cell CD137 binder Antigen binder Linker Nov-19 Nectin-4/CD137 TICA molecules activate human PBMCs in a Nectin-4 dependent manner CD137 provides robust anti-tumor T cell and NK cell effects Nectin-4 Bicycle CD137 Bicycle -11 -10 -9 -8 -7 -6 0 5000 10000 15000 20000 25000 30000 4T1-Parental Log concentration (M) I F N g ( p g / m l ) -11 -10 -9 -8 -7 -6 0 5000 10000 15000 20000 25000 30000 4T1-D02 (Nectin4+) Log concentration (M) I F N g ( p g / m l ) BCY10000 (Nectin-4/CD137) BCY9350 (Nectin-4(nb)/CD137) BCY9351 (Nectin-4/CD137(nb)) -11 -10 -9 -8 -7 -6 0 500 1000 1500 2000 2500 Log concentration (M) I L - 2 ( p g / m l ) -11 -10 -9 -8 -7 -6 0 500 1000 1500 2000 Log concentration (M) I L - 2 ( p g / m l )
16 Dosing of BT7480 leads to robust anti-tumor activity and immune memory in a syngeneic model Rechallenge of “cured” mice with tumor reimplantation shows no tumor growth, implying immunogenic memory Nectin-4-CD137 TICATM achieves rapid tumor regression Nov-19 0 7 14 21 28 0 500 1000 1500 2000 MC38 #13 in huCD137 C57Bl/6 mice T u m o r v o l u m e ( m m 3 , m e a n + / - S D ) * *** *** BCY11863 10mg/kg Q3D BCY11863 10mg/kg QD Vehicle BCY11863 1mg/kg Q3D BCY11863 1mg/kg QD *** p £ 0.0001, 2W-ANOVA with Dunnett's ** p £ 0.01 * p £ 0.05 ** * * ** ** * *** *** *** * ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ ^ Day ^ QD Q3D Dosing 0 7 14 21 0 200 400 600 800 1000 Re-challenge of CR mice with MC38#13 cells Days after cell implantation T u m o r v o l u m e ( m m 3 ) Control huCD137-C57Bl/6 mice (n=5) BCY11863 CR mice (n=5)

17 Immune cell engaging Bicycles® and tumor antigen engaging Bicycles can be readily interchanged OX40 Bicycle Nectin-4 Bicycle 4T1 (+ve) Nectin-4 4T1 (-ve) Nectin-4 D1 D2 D3 Nov-19 CD137 TICATM (BCY10000) OX40 TICA (BCY12141) EphA2 TICA Potential to rapidly and efficiently generate multiple clinical candidates CD137 Bicycle EphA2 Bicycle CD137 Bicycle Nectin-4 Bicycle -11 -10 -9 -8 -7 -6 0 2000 4000 6000 8000 10000 Log concentration (M) I L - 2 ( p g / m L ) b a c k g r o u n d s u b t r a c t e d -11 -10 -9 -8 -7 -6 0 1000 2000 3000 Log concentration (M) I L - 2 ( p g / m L ) BCY12141 (4T1 Nectin-4 +ve) BCY12141 (4T1 Nectin-4 -ve) No treatment -11 -10 -9 -8 -7 -6 0 5000 10000 15000 Log concentration (M) I F N ? ( p g / m L ) BCY12759 (4T1 Nectin-4 -ve) BCY10575 (4T1 Nectin-4 +ve)

Beyond Oncology

Partnerships with leading therapeutic experts to explore broad application of Bicycles® beyond oncology Nov-19 19 Bicycle’s unique technology & deep technical understanding Development & therapeutic expertise Differentiated treatments for diseases with high unmet need + TREATMENTS PARTNERS

THR-149: Novel Pkal inhibitor provides validation of Bicycle® platform for ophthalmological diseases 20 Phase I trial evaluated safety of a single intravitreal injection of THR-149 at 3 ascending dose levels in 12 patients with visual impairment due to center-involved diabetic macular edema (DME) No dose-limiting toxicities or drug-related serious adverse events reported Topline data show that THR-149 is well-tolerated and safe Increasing average improvement in BCVA of up to 7.5 letters at Day 14 following a single injection of THR-149 Rapid onset of action starting at Day 1 An average improvement in BCVA of 6.5 letters at Day 90 following a single injection of THR-149 Activity maintained *BCVA = Best Corrected Visual Acuity Nov-19

Establishing Bicycle as an integrated, top tier biotech company Nov-19 21 BT1718 validates approach, PK, tolerability, tumor accumulation Emerging preclinical data establishes IO opportunity BTCs validated as monotherapy option BT7480 establishes translatability of TICA™ approach Collaborations established in key therapeutic areas Preclinical and clinical validation Internal programs Combination approaches within and outside Bicycle pipeline to address key unmet needs in oncology Develop multi-asset portfolio with significant clinical potential ONCOLOGY NON-ONCOLOGY TODAY NEAR TERM MID TERM

Bicycle Therapeutics is led by an experienced team, growth is enabled by robust financial profile Nov-19 22 *As of September 30, 2019 Clinical data readouts at medical meetings and trial initiations expected in 2020 across BTC, IO and partnered programs 2020 Key Events BT1718 PhIIa start BT5528 interim PhI data BT8009 PhI start BT7480 IND enabling activities Experts in drug development with executive experience at leading companies Cash balance of $96.0M* provides runway to support multiple clinical milestones

Thank you

Appendix

25 Chemical nature of platform allows rapid “dialing in” of most desirable properties 25 EC50 (nM) >90 Nectin-4 TICA molecules synthesized in combinatorial manner Size of sphere proportional to molecular weight 1:2 Bicycles 1:1 Bicycles® 1:3 Bicycles Reporter cell assay data for 30 Nectin-4/CD137 TICAs in co-culture with HT1376 CD137L Nov-19 ONCOLOGY BEYOND ONCOLOGY + Loop 1 Loop 2 N-term Cys Lys3 dLys4 Loop 1 Loop 2 C-term CD137 Monomers x10 4 Attachment pts Linkers enable various architectures 1:1 1:2 1:3 Nectin-4 Monomers x6 2 Attachment pts dLys3 N-term > 60 TICAs™ synthesized 1:1 1:2 1:3 10 100 0.01 0.1 1 10 100


